
 |
|
|
|
|
R. A. Jonas, MD [MEDLINE LOOKUP]
Boston [MEDLINE LOOKUP]
Mass. [MEDLINE LOOKUP]
J. M. Quaegebeur, MD [MEDLINE LOOKUP]
New York [MEDLINE LOOKUP]
N.Y. [MEDLINE LOOKUP]
J. W. Kirklin, MD [MEDLINE LOOKUP]
E. H. Blackstone, MD [MEDLINE LOOKUP]
Birmingham [MEDLINE LOOKUP]
Ala. [MEDLINE LOOKUP]
G. Daicoff, MD [MEDLINE LOOKUP]
St. Petersburg, Fla., and the Congenital Heart Surgeons Society

Patients and Methods 
Results 
Discussion

Among 183 neonates with interrupted aortic arch and ventricular septal defect entering a multiinstitutional study between 1987 and 1992, nine died before repair was accomplished. Among the remaining 174, survival at 1 month and 1, 3, and 4 years after repair was 73%, 65%, 63%, and 63%, respectively. The risk factors for death were low birth weight, younger age at repair, interrupted arch type B, outlet and trabecular ventricular septal defects, smaller size of the ventricular septal defect, and subaortic narrowing. Echocardiographically measured dimensions (expressed as Z-values) at all levels of the left heartaorta complex were small. Two among thirty institutions were risk factors, and two others possibly were. Procedural risk factors for death after repair were (1) repair without concomitant procedures in patients with other important levels of obstruction in the left heartaorta complex, (2) a Damus-Kaye-Stansel anastomosis, and (3) subaortic myotomy/myectomy in the face of subaortic narrowing. One-stage repair plus ascending aorta/arch augmentation had the highest predicted time-related survival in the 20% of patients with interrupted aortic arch and one or more coexisting levels of obstruction in the left heartaorta complex, as did initial repair without or with aorta/arch augmentation in the 80% without these. (J THORAC CARDIOVASC SURG 1994;107:1099-113)
Patients
By chance, precisely 250 neonates with IAA entered into the institutions between January 1, 1987, and January 1, 1992 ( Appendix Table 1).
| Total | |||||
| Total deaths | |||||
| Coexisting cardiac anomalies | n | % of 250 | No. | % | 70% CL (%) |
| VSD | 183 | 73 | 71 | 39 | 35-43 |
| Truncus arteriosus | 25 | 10 | 17 | 68 | 56-79 |
| AP window | 10 | 4 | 1 | 10 | 1-30 |
| Univentricular AV connection | 9 | 4 | 6 | 67 | 44-85 |
| TGA with VSD | 8 | 3 | 3 | 38 | 17-62 |
| DORV | 5 | 2 | 3 | 60 | 29-86 |
| Taussig-Borg DORV | 4 | 2 | 3 | 75 | 37-97 |
| Complete AV Canal | 1 | 0.4 | 1 | 100 | 15-100 |
| Corrected Transposition | 1 | 0.4 | 0 | 0 | 0-85 |
| None | 4 | 2 | 1 | 25 | 3-63 |
| Total | 250 | 100 | 106 | 42 | 39-46 |
AP, Aortopulmonary; AV, atrioventricular; DORV, double-outlet right ventricle; TGA, transposition of the great arteries. |
|||||
*Double-inlet left ventricle in three; mitral atresia in four; tricuspid atresia in two. |
|||||
Other potentially obstructive levels in the left heartaorta complex
An important consideration is the possible coexistence elsewhere in the left heartaorta complex of hypoplasia or other anomalies that are potentially obstructive. These include mitral valve anomalies, left ventricular hypoplasia, subaortic narrowing, left ventricularaortic junction narrowing (anulus), aortic valvular narrowing, and hypoplasia of ascending aorta (including the sinuses of Valsalva) and proximal arch (proximal to the origin of the left common carotid artery). The distal arch (beyond the origin of the left common carotid artery but proximal to the attachment of the ductus arteriosus) is usually the site of the interruption. Pressure gradients are of little value in assessing the functional severity of potential obstruction at these levels in newborn infants, because in them blood flow through at least some parts of the left heartaorta complex is reduced by patency of the ductus arteriosus, a VSD, or similar conditions.
Echocardiographic measurements
Echocardiographic measurements of the dimensions (usually diameters in millimeters) of one or more of the various levels in the left heartaorta complex were recorded preoperatively by some institutions for some patients. Only the diameters of the subaortic area, the left ventricularaortic junction (anulus), and the ascending aorta were recorded in sufficient frequency by the institutions to justify consideration of their use in this study. The diameters were standardized to Z-values 2 (standard deviation units) by the equation:
Z = Observed dimension Mean normal dimension/Standard deviation of the mean normal dimension
The normal refers to the value of the dimension in normal individuals of the same body surface area as the patient, computed by previously generated regression equations. 2-4
Precise and appropriately standardized, normalized, and studied measurements should provide the best information about the resistance to forward flow. However, many patients in this study were without echocardiographic measurements, and the echocardiographic techniques of measurement were not well standardized. Therefore, in the face of no better alternative, and recognizing the imperfections in the method adopted, an overall estimate has also been made of the prerepair resistance to forward flow in each of the possibly obstructive levels in the left heartaorta complex, grading this as 0 to 5, with 5 indicating severe resistance. Grade 2 or greater resistance (>grade 2) has been considered to be functionally significant.
Follow-up
A cross-sectional follow-up of all patients was performed annually. Eighty percent of the patients were followed up by the Data and Analysis Center, and the remainder were followed up by the treatment institution, at the institutions request. The last follow-up was conducted between March 1 and May 1, 1992, with additional follow-up in September and October, 1992, of recently discovered cases.
Among the 174 patients, 62 were known to be dead before the initiation of the last follow-up; an attempt was made to trace the other 112 at this follow-up. This was unsuccessful in one who has been lost to follow-up since 1988, in one who has been lost since 1989, and in three lost since 1990. In addition to these five patients, eight who were followed up in 1991 could not be traced. Thus 13 (7.5%) of the 174 patients not known to be dead are without a current follow-up.
In all, the 90th, 75th, 50th, 25th, and 10th percentiles of patients have been followed 7.4, 13.2, 22.8, 36.7, and 54.4 months since repair, respectively. The range is 13 days to 63.1 months. Mean follow-up time for surviving patients is 26.2 ± 16.82 (standard deviation) months.
Database
Copies of parts or all of the hospital records were provided to the Data and Analysis Center in the Division of Cardiothoracic Surgery at the University of Alabama at Birmingham. These contained the basic information about the patients and their hospitalizations. The information on each patient was entered into a computer database and rechecked and upgraded on numerous occasions. Both noncomputerized and computerized files were treated in a highly confidential manner.
Analysis
A dataset for analysis was created from the computer database, using SAS System software (SAS Institute, Inc., Cary, N.C.) and an IBM RISC computer (IBM Corp., Atlanta, Ga.).
Numerous explorations were made by means of Kaplan-Meier time-related estimations, 5 cumulative frequency distribution plots, and contingency tables. P values were estimated, and the method (model) is given wherever these are shown. Survival and time-related freedom from other unfavorable outcome events, and their hazard functions, also were estimated parametrically by means of the hazard function regression model. 6 Multivariable analyses were performed in this domain (see Appendix 2).
Validations of the multivariable risk factor equations were performed by means of the method described for the logistic regression domain by Hosmer and Lemeshow. 7 The basic theorem of the method was proved by Turner (Turner ME: personal communication; 1985), which allowed its adaptation to the hazard function regression method. 8,9
Non-risk-adjusted prevalences of death (or survival) and other events are those that actually pertained. They are obtained by simply counting and dividing or by actuarial analysis. These are usually of limited value in drawing inferences, because of the heterogeneity of the groups, even when stratified by the value of some variable. Risk-adjusted prevalences or rates are those in which the values for all variables except the one(s) under consideration are kept constant; that is, the effect of the variable(s) under consideration is isolated from the effect of any other variables and thus is more clearly evident. Risk-adjusted prevalences are valuable in drawing inferences, but there is always a degree of uncertainty (quantified by confidence intervals and P values) in their values.
Survival
Time-related survival among the 174 neonates with IAA and VSD who underwent arch repair (with or without concomitant closure of the VSD or a procedure against one or more obstructive levels in the left heartaorta complex) is depicted in Fig. 1, A.
| Fig. 1. Non-risk-adjusted survival and hazard function for death. A, Survival after the initial arch repair (at time zero). Each circle represents an actual death, positioned at the time of death along the horizontal axis and actuarially along the vertica laxis. The vertical bars depict ±1 standard error. The numbers indicate the number of patients remaining at risk at the time of the estimate. The solid line is the parametric estimate of survival, and the dashed lines enclose the 70% confidence intervals. The dashed-dot-dashed line represents the survival of an age-gender-matched general population (U.S. life tables). B, Hazard function for death. |
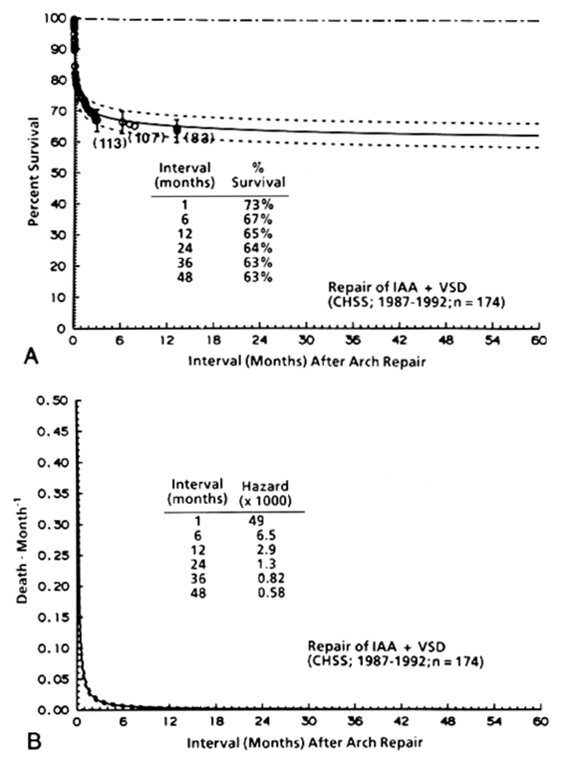 |
The hazard function for death has only a single declining phase, with a sharp change (inflection) in the rate of decline about 2 months after repair ( Fig. 1, B).
Demographic information
The 50th percentile of ages at entry was 2.4 days, and that of birth weight was 3.1 kg. The median interval between time of entry and time of repair was 3 days.
Both smaller birth weight and younger age at repair were incremental risk factors for death at any time after repair ( Table I).
| Incremental risk factors for death (patient-specific variables) | Pvalue (single hazard phase) |
| Demographic | |
| Birth weight* (lower) | <0.0001 |
| Age at repair* (younger) | 0.004 |
| Morphologic | |
| IAA type B | 0.02 |
| Outlet or trabecular VSD | 0.0003 |
| Size of VSD (small, moderate-sized, large)** (smaller) | <0.0001 |
| Subaortic narrowing (grade 0-5)** (greater) | 0.0004 |
IAA, Interrupted aortic arch; VSD, ventricular septal defect. |
|
Note: (1) In this and all of the other multivariable equations, the variables without asterisks are categorical (yes/no) ones. One asterisk (*) indicates continuous variables; two asterisks (**) indicate ordinal variables. (2) In this and all of the other multivariable equations, mitral valve anomalies (three patients, two of whom died) were computationally intractable to iterative estimations and therefore are not included in the equation; they are possibly risk factors. |
|
Morphology
Seventy-nine percent of the patients undergoing repair had IAA type B; the remainder had type A. Type B resulted in the highest proportion of total non-risk-adjusted deaths (P[x 2] = 0.009) and was also a risk factor for death by multivariable analysis ( Table I).
Anomalous origin of the right subclavian artery (from the upper descending thoracic aorta) occurred in 29% of patients, and DiGeorge syndrome occurred in 27%; they uncommonly occurred in the same patient and both occurred dominantly but not exclusively in patients with IAA type B. Neither increased the non-risk-adjusted or the risk-adjusted probability of death after repair in this study.
The VSD was most commonly of the conoventricular type 10 ( Appendix Table 3).
| Total deaths | ||||||
| Location of VSD | n | % of 166 | No. | % | 70% CL (%) | |
| Conoventricular | 129 | 78 | 39 | 30 | 26-35 | P( 2) = 0.008 2) = 0.008 |
| Outlet* | 24 | 14 | 14 | 58 | 46-70 | |
| Trabecular | 9 | 5 | 4 | 44 | 24-66 | |
| Inlet | 4 | 2 | 2 | 50 | 18-82 | |
| Subtotal | 166 | 100 | 59 | 36 | 32-40 | |
P( 2) 2) |
0.05 | |||||
| Unknown | 8 | 3 | ||||
| Total | 174 | 62 | 36 | 32-40 | ||
CL, Confidence limits. |
||||||
*Juxtapulmonary or juxtaarterial. When severe conal (infundibular) septal hypoplasia is present, this type of defect may be difficult to distinguish from conoventricular VSD. |
||||||
Eight neonates had multiple VSDs; three (38%; CL 17% to 62%) of these died. |
||||||
Other obstructive levels in the left heartaorta complex |
||||||
Other obstructive levels in the left heart-aorta complex.
One or more additional anomalies of the left heartaorta complex resulting in a functionally important impediment to flow (see definitions in Patients and methods) were present in 33 patients (21% of the 159 in whom this could be determined; Fig. 2).
The non-risk-adjusted prevalence of total deaths (52%) was believably higher in these patients (P[ 2] = 0.03). However, subaortic or annular arrowing (present in 26 of the 33 patients) was the only one of these appearing as an incremental risk factor by simultaneous multivariable analysis (see
2] = 0.03). However, subaortic or annular arrowing (present in 26 of the 33 patients) was the only one of these appearing as an incremental risk factor by simultaneous multivariable analysis (see Table I).
The absence of parachute mitral valve (with single papillary muscle) in this group of patients is noteworthy, considering its prevalence in neonates entering for treatment because of coarctation (Quaegebeur JM, Jonas RA, Weinberg AD, Blackstone EH, Kirklin JW and the Congenital Heart Surgeons Society: unpublished data, 1993).
Echocardiographic measurements
The dimensions (Z-values) of the subaortic channel, the left ventricularaortic junction (aortic anulus), and the ascending aorta, measured at the patients institutions, were in general small (Z-values <2.0). All were smaller than in patients with coarctation, but only that of the ascending aorta was believably so (Appendix Fig. 1). The very small actual diameter implied by these small Z-values is shown in
Table II.
| Left ventricular-aortic junction (diameter) | |
| Millimeters | Z-value |
| 9.7 | 0 |
| 8.6 | 1 |
| 7.6 | 2 |
| 6.7 | 3 |
| 5.9 | 4 |
| 5.2 | 5 |
| 4.6 | 6 |
| 4.1 | 7 |
| 3.6 | 8 |
| 3.2 | 9 |
Note: The difference between Z of 3 and of7, for example, is only 2.6 millimeters. |
|
| Incremental risk factors for death (patient-specific variables, including institutionally measured dimensions) | P value (single hazard phase) |
| Demographic | |
| Birth wieght* (lower) | <0.0001 |
| Age at repair* (younger) | 0.04 |
| Morphologic | |
| IAA type B | 0.02 |
| Outlet or trabecular VSD | 0.003 |
| Size of VSD** (smaller) | 0.0002 |
| Dimension (Z) of LV-aortic junction* (smaller) | 0.03 |
LV, Left ventricular. |
|
Note:For this, the obstructive levels in theleft heart-aorta complex were not entered. The information content of this equation is less thanthat of the equation in Table I, possibly because of the relatively small number of patients with measured dimensions. |
|
*Continuous variables. |
|
**Ordinal variables. |
|
Institutional differences
Non-risk-adjusted survival of the neonates varied widely, according to the institution in which they were treated ( Appendix Table 5).
| Total deaths | ||||
| Institution of repair (coded) | n | No. | % | 70% CL (%) |
| CC | 3 | 0 | 0 | 0-47 |
| Z | 2 | 0 | 0 | 0-61 |
| EE | 1 | 0 | 0 | 0-85 |
| DD | 1 | 0 | 0 | 0-85 |
| N | 1 | 0 | 0 | 0-85 |
| X | 10 | 1 | 10 | 1-30 |
| AA | 8 | 1 | 12 | 2-36 |
| W | 5 | 1 | 20 | 3-53 |
| Y | 5 | 1 | 20 | 3-53 |
| U | 9 | 2 | 22 | 8-45 |
| Q | 4 | 1 | 25 | 3-63 |
| R | 4 | 1 | 25 | 3-63 |
| BB | 4 | 1 | 25 | 3-63 |
| V | 22 | 6 | 27 | 17-40 |
| L | 10 | 3 | 30 | 14-51 |
| T | 6 | 2 | 33 | 12-62 |
| O | 3 | 1 | 33 | 4-76 |
| P | 8 | 3 | 38 | 17-62 |
| S | 17 | 7 | 41 | 27-57 |
| J | 4 | 2 | 50 | 18-82 |
| I | 2 | 1 | 50 | 7-93 |
| F | 2 | 1 | 50 | 7-93 |
| G | 2 | 1 | 50 | 7-93 |
| K | 19 | 10 | 53 | 38-66 |
| H | 10 | 6 | 60 | 39-78 |
| D | 3 | 2 | 67 | 24-96 |
| B | 5 | 4 | 80 | 47-97 |
| C | 2 | 2 | 100 | 39-100 |
| M | 1 | 1 | 100 | 15-100 |
| E | 1 | 1 | 100 | 15-100 |
| Total | 174 | 62 | 36 | 32-40 |
CL, Confidence limits. |
||||
| Incremental risk factors for death (patient-specific and institutional variables) | P-value (single hazard phase) |
| Demographic | |
| Birth weight* (lower) | <0.0001 |
| Age at repair* (younger) | 0.002 |
| Morphologic | |
| IAA type B | 0.02 |
| Outlet or trabecular VSD | 0.003 |
| Size of VSD** (smaller) | 0.0001 |
| Subaortic narrowing (grade 0-5)** | 0.006 |
| Institutional | |
| Institution B | 0.006 |
| Institution H | 0.0006 |
IAA, Interrupted aortic arch; VSD, ventricular septal defect. |
|
Note: The Q-statistics coefficient for institution M is 7.8, P = 0.009 (one case, one death), and for institution C it is 7.4, P= 0.0003 (two cases, two deaths). Computational intractability precluded iterative estimations for these institutions as risk factors, and therefore they are not included in the risk factor equation; possibly they are risk factors. |
|
* Continuous variables. |
|
** Ordinal variables. |
|
| Incremental risk factors for death (patient-specific, procedural, and institutional variables) | P value (single hazard phase) |
| Demographic | |
| Birth weight* (lower) | <0.0001 |
| Age at repair* (younger) | 0.0004 |
| Morphologic | |
| Grade of subaortic obstruction (0-5) (higher) | 0.0004 |
| IAA type B | 0.04 |
| Size of VSD (small, moderate-sized, large)** (smaller) | <0.0001 |
| Procedural | |
| PT-Asc Ao anastomosis (DKS anastomo-sis) | <0.0001 |
| Subaortic myotomy/myectomy and sub-aortic obstruction (grade 2) (interac-tion term) | 0.02 |
| Simple repair and coexisting obstructive lesions elsewhere in the LHA complex (interaction term) | 0.02 |
| Institutional | |
| Institution B | 0.006 |
| Institution H | <0.0001 |
DKS Damus-Kay-Stansel; IAA, Interrupted aortic arch; LHA, left heart-aorta. |
|
Note: The Q-statistics coefficient for institution C is 5.7, P = 0.002 (two cases, two deaths). Computational intractability precluded iterative estimation of this institution as a risk factor, although it might be. |
|
*Continuous variables. |
|
**Ordinal variables. |
|
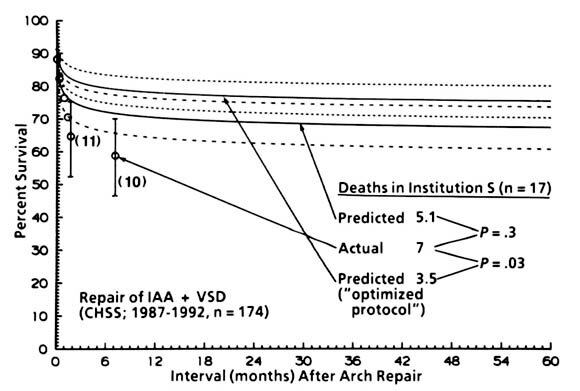
| Appendix Fig. 2. Non-risk-adjusted actuarial survival (labeled actual) by the Kaplan-Meier method, and total deaths in institution S; also, survival and total deaths in this institutions very patients are shown as predicted by the equation in Appendix Table 6. In addition, survival and total deaths are predicted according to the same equation and for the same patients in institution S but entering the values for the procedural variables all as no; this solution is labeled predicted ("optimized protocol"). The inference is that low-risk institution S could have still further improvement in results by using an "optimized protocol," in which only arch augmentation is used for patients with coexisting obstructive lesions elsewhere in the left heart-aorta complex, or simple repair when there are no such coexisting lesions. CHSS, Congenital Heart Surgeons Society. |
 |
The effect of the initial procedure
Only 26% of patients in whom a lateral thoracotomy was used (as in most initial repairs in which the VSD was not closed) had a direct anastomosis, in contrast to 92% of those in whom a median sternotomy and a one-stage repair was performed.
The small differences between the non-risk-adjusted prevalence of total deaths after initial one-stage repair and those after an initial arch repair in which the VSD was not closed are possibly due to chance alone ( Table IV).
| Total deaths | ||||||
| First repair* | n | No. | % | 70% CL (%) | ||
| One-stage repair | 116 | 44 | 38 | 33-43 | P( 2) = 0.7 2) = 0.7 |
|
| Repair IAA + PT band | 40 | 14 | 35 | 27-44 | ||
| Repair only IAA | 17* | 4 | 24 | 12-39 | P( 2) = 0.5 2) = 0.5 |
|
| Transplant | 1 | 0 | 0 | 0-85 | ||
| Subtotal | 174 | 62 | 36 | 32-40 | ||
P( 2) 2) |
0.6 | |||||
| No repair of anything | 9 | 9 | 100 | 81-100 | ||
| Total | 183 | 71 | 39 | 35-43 | ||
CL, Confidence limits, IAA, interrupted aortic arch; PT, pulmonary trunk. |
||||||
*Seven (zero deaths) had type A interruption; 10 (four deaths) had type B interruption. |
||||||
| Total deaths | ||||
| Concomitant procedures against other obstructive lesions at initial operation | n | No. | % | CL (%) |
| Asc Ao/arch | 20 | 4 | 20 | 10-33 |
| augmentation* | ||||
| Myotomy/myectomy | 15 | 7 | 47 | 31-63 |
| PT-Asc Ao anastomosis | 11 | 10 | 91 | 72-99 |
| (DKS) | ||||
| Closed surgical aortic | 1 | 0 | 0 | 0-85 |
| valvotomy | ||||
| Tube graft from PT to | 3 | 1 | 33 | 4-76 |
| Desc Ao | ||||
| Transplantation | 1 | 0 | 0 | 0-85 |
| None | 122 | 40 | 33 | 28-38 |
| Total | 173! | 62 | 36 | 32-40 |
Asc Ao, Ascending aorta; Desc Ao, descending aorta; DKS, Damus-Kaye-Stansel; PT, pulmonary trunk. |
||||
*Ascending aorta/arch augmentation was usually accomplished with pericardium or allograft material for patch-graft augmentation of alarge side-to-end anastomosis incorporating extensions of the aortotomy into the left common carotid artery proximally and the left subclavianartery distally, as proposed by Edmunds, Norwood, and Low.11 |
||||
Nine of the 11 procedures were classic Damus-Kaye-Stansel end-to-side anastomoses; two consisted of banding of the pulmonary trunk and a side-to-side anastomosis between the ascending aorta and pulmonary trunk, using an interposed tube-graft for one. |
||||
!One additional patient (who lived) had both ascending aorta/arch anastomosis augmentation and myotomy/myectomy. |
||||
Interestingly, over half of the patients receiving such concomitant procedures appear retrospectively to be without obstructive lesions other than at the IAA. However, among 11 patients with these coexisting obstructive lesions but without a concomitant procedure of some sort directed against one or more of them, six (55%) died.
Thus percent survival in patients with IAA and VSD only (without other coexisting obstructive lesions in the left heartaorta complex) was highest among those undergoing a repair of the IAA without a concomitant procedure or with only ascending aorta/arch augmentation (see Fig. 4). In the 20% of patients in whom obstruction existed elsewhere in the left heartaorta complex, the percent survival was highest among those undergoing ascending aorta/arch augmentation.
Delayed closure of the VSD
Among the 57 patients who did not have the VSD closed at the initial procedure, essentially all who survived had it closed, nearly always by surgery, within 36 months of the initial repair. The peak rate of closure (hazard function) by a subsequent procedure was highest during the first month after the initial procedure, suggesting that the open VSD was not being well tolerated and that a one-stage repair at the initial procedure might have been preferable.
Reinterventions against the repair of the IAA by one-stage repair
Twenty patients underwent reintervention against the arch repair itself, and in nine the procedure was percutaneous balloon dilation. The peak of the hazard function for this reintervention was at about 4 months after the initial repair.
Reintervention against coexisting obstructive lesions in the left heart-aorta complex
Fifteen of the 116 patients undergoing one-stage repair had a first reintervention against one or more coexisting obstructive lesions in the left heartaorta complex ( Table VII), and the time-related freedom from such reintervention was only 77% at 3 years (
Fig. 5).
| Total deaths | |||
| Type of first reintervention against other obstructions | n | No. | % |
| Asc Ao/arch augmentation | 5 | 3 | 60 |
| Subaortic myotomy/myectomy | 2 | 0 | 0 |
| Asc Ao/arch augmentation + myectomy | 1 | 0 | 0 |
| LV-aortic conduit | 1 | 1 | 100 |
| Surgical aortic valvotomy | 1 | 0 | 0 |
| Percutaneous balloon aortic valvotomy | 5 | 0 | 0 |
| Total | 15 | 4 | 27 |
Asc Ao, Ascending aorta; LV, left ventricle. |
|||
Note: No patient received a Damus-Kaye-Stansel operation as a first reintervention. |
|||
One patient (who lived) underwent a Konno operation with an allograft aortic valve as a second reintervention, another (who lived) underwent a myotomy/myectomy and aortic valvotomy as a second reintervention, and a third (who lived) underwent ascending aorta/arch augmentation and myotomy/myectomy as a second reintervention.
One inference from the multivariable risk factor equation for this type of reintervention as an outcome event ( Table VIII) is that interventions of these types are less prevalent in patients with coexisting obstructive lesions undergoing an initial one-stage repair when a concomitant procedure was performed at the initial repair.
| Incremental risk factors for reinterventions against other obstructive lesions after initial one-stage repair | Pvalue (single hazard phase) |
| "Simple" initial repair and presence of coex-istingother obstructions in the LHA com-plex (interaction term) | <0.0001 |
| No myotomy/myectomy and subaortic nar-rowing grade 2 at initial repair (interaction term) | 0.02 |
LHA, Left heart-aorta. |
|
Note: "No myotomy/myectomy" is undesirable in form, but "subaortic myotomy/myectomy" made the interaction term computationally intractable; there were no (reinterventions) in nine such patients (four of whom died). |
|
Potentially optimal results with current knowledge
A patient with only IAA type B and VSD, undergoing one-stage repair with no concomitant procedure or with only ascending aorta/arch augmentation, is predicted to have a 1-month survival of 89% and a 5-year survival of 83%; survival in type A is somewhat better ( Fig. 6).
Representativeness of the sample in the study
The young age (2.4 days) of the patients on admission suggests that the patients are a reasonably representative sample of newborn infants with IAA and VSD. However, the fact that nine (approximately 5%) of the 183 patients entering the hospital died shortly after admission and before an operation was accomplished suggests that at least another 5% to 10% of those born in the regions served by the hospitals died before admission, and the presumption is that the anomaly was more severe or the ductus arteriosus closed sooner in these patients.
The number of neonates entering the Congenital Heart Surgeons Society institutions who were not entered into the study is not known. However, in view of the detailed and repeated measures taken to ensure capturing all entrants, it is believed that nearly all those entering the institutions were included in the database.
Validity of the multivariable equations
The good correspondence between the actual number of total deaths in the various stratified groups and the number of deaths predicted by patient-specific solutions of the multivariable equations, and the good correspondence between the Kaplan-Meier time-related depictions of percent freedom from an event and the time-related patient-specific predictions of the percent freedom (seven such comparisons were made, for example see Figs. 2 and 4) indicate the internal validity of the multivariable equations. The predictive validity is of course not determined by these tests but will be evaluated by validation tests of prediction of outcomes in future patients; such studies in the past have validated the predictive value of similar equations. 12
Obstructions at other levels in the left heartaorta complex
The fate of surgically untreated obstructions at other levels, such as in the left ventricular outflow tract (subaortic channel, anulus, or aortic valve) or the ascending aorta or arch, is not known. It should be determinable by serial echocardiographic measurements before and early and late after initial repair without a procedure directed against any obstructive lesion of the left heartaorta complex. The fact that relatively few patients (about 20% of surviving patients, see Fig. 5) required reintervention directed against these areas, which are known usually to be small in neonates with IAA, suggests that they do enlarge rather rapidly after repair in most patients.
The great value of echocardiographic measurements, both in directing the details of initial surgical treatment and in following the postoperative growth at all levels in the left heartaorta complex, emphasizes the importance of achieving a standardized echocardiographic technique, so that adequate comparisons can be made.
Inferences as to the nature of IAA
In general, all dimensions in the left heartaorta complex are small in patients with IAA and VSD, and there is no certainty that this is different in the two types of IAA. The Z-values of the dimensions of the subaortic area tend to be the smallest of those of any area (see Appendix Fig. 1). When subjectively evaluated, subaortic narrowing also appears to be the commonest important coexisting impediment to left ventricular inflow or outflow. 13 Such impediments in the mitral area are uncommon.
It follows that many patients with IAA have a series of resistances to left ventricular outflow, beginning with those in the subaortic channel and including those in the left ventricularaortic junction (anulus) and the aortic valve, the ascending aorta, the proximal arch (distal to the innominate artery but proximal to the left common carotid artery), and the distal arch (distal to the left common carotid artery and proximal to the aortic attachment of the ductus arteriosus).
The small size at these levels may be the developmental result of a large VSD and patency of a large ductus arteriosus supplying blood to the descending thoracic aorta. By analogy with other areas of the circulation (for example, the distal aortic arch after repair of coarctation 14 or the pulmonary arteries in tetralogy of Fallot with pulmonary atresia after a procedure to increase pulmonary blood flow 15,16), these areas may enlarge immediately and grow disproportionally after a reparative procedure that obligates them exclusively to transport the systemic blood flow to all areas beyond them. However, one or more of the areas may have, in addition, structural wall abnormalities that prevent both immediate enlargement and subsequent disproportionate growth from occurring, although currently this cannot be determined except by observation after repair. It does appear that, in some areas (at least, the left ventricle, subaortic channel, and mitral valve), the smaller the dimensions, the less likely is it that one or both of these favorable sequelae will occur with sufficient rapidity to allow survival.
Inferences as to therapy:
In neonates with only IAA type B and VSD, one-stage repair without any concomitant procedure or with only ascending aorta/arch augmentation offers the patient a good chance (±85%) for survival for at least 5 years. In addition, when augmentation is used, the probability is high (85%) that no reintervention will be required against any other obstructive lesion of the left heartaorta complex. Some possibility (±6%) exists that reintervention against the aortic anastomosis will be required, but this can often be performed by percutaneous techniques with low risk.
In patients with apparently important subaortic narrowing, one-stage repair with subaortic myotomy/myectomy has given only about a 50% probability of survival for 5 years in the Congenital Heart Surgeons Society experience but very little probability of the need for reintervention. Bove, 17 Ilbawi, 18 and their colleagues have reported better results in small groups of patients. However, ascending aorta/arch augmentation at the time of one-stage repair achieves the same very low probability of reintervention and gives, in this experience, about an 80% predicted survival for at least 5 years. During this repair, outlet VSDs may advantageously be closed by approaching them through the pulmonary trunk.
Of considerable interest is the fact that ascending aorta/arch augmentation is as effective as any other concomitant procedure in maximizing survival after initial repair of IAA in patients with coexisting obstructive lesions in the left heartaorta complex. The explanation of this may be that relief of that component of the total gradient caused by the narrowing ascending aorta and arch is sufficient to allow survival of the patient even though that from coexisting subaortic narrowing may persist for at least a time after repair.
Reintervention against subaortic narrowing, when shown by follow-up studies and the condition of the patient to be necessary, can probably be accomplished as early as a few weeks after the initial operation, although it possibly should be delayed if the patients condition allows it. This reintervention could consist of (1) simple resection of the subaortic narrowing through the aortic root, (2) a Vouhé aortoseptal approach, 19 or a modified Konno approach 20 to subaortic resection and/or patch graft enlargement, or (3) a Konno operation with subaortic resection and/or patch graft enlargement and reconstruction with a pulmonary valve and artery autograft or appropriate-sized aortic valve and ascending aortic allograft. 21,22
A few patients with important hypoplasia of the left ventricle (or surgically uncorrectable anomalies of the mitral valve, rare in patients with IAA) probably require a one-ventricle repair. When the indications are strong that this is the case, the initial procedure may need to be a Norwood operation (of the type used for the first stage of the repair of aortic atresia 23) and subsequently a partial or complete Fontan operation or, alternatively, cardiac, ascending aorta, and arch transplantation. 24,25
We express our appreciation of the work of the pediatric cardiologists, cardiac surgeons, nurses, and coordinators in the institutions participating in this study of the Congenital Heart Surgeons Society. The institutions, in randomly determined order, are Mott Childrens Hospital at the University of Michigan Medical Center; University of Alabama Medical Center; The Boston Childrens Hospital; The Childrens Hospital in Buffalo, New York; University of Chicago; Childrens Memorial Hospital in Chicago; Childrens Hospital of Michigan in Detroit; The Penn State College of Medicine (The Milton S. Hershey Medical Center); University of Iowa Hospital and Clinics; Childrens Hospital of Los Angeles; Miami Childrens Hospital; University of Miami (and Jackson Memorial Hospital) in Miami; The Montreal Childrens Hospital; Columbia-Presbyterian Medical Center in New York; Childrens Hospital of Philadelphia; University of California San Francisco; University of Utah Medical Center (and Primary Childrens Medical Center); All Childrens Hospital in St. Petersburg, Florida; Hospital for Sick Children in Toronto; University of California Los Angeles; British Columbia Childrens Hospital in Vancouver; University of São Paulo; Childrens Hospital Medical Center, Cincinnati; Christ Hospital and Medical Center, Oak Lawn, Illinois; Loma Linda University Medical Center, Loma Linda, California; University of Nebraska Medical Center and Childrens Memorial Hospital, Omaha, Nebraska; Childrens Hospital of Pittsburgh; Medical University of South Carolina; Childrens Hospital and Health Center, San Diego; St. Christophers Hospital for Children, Philadelphia. We also thank Mary Lynne Clark, Phyllis Newsom, and Gail Mertz for their skill and diligence in obtaining the follow-up information. Rob Browns skillful data management and analyses are acknowledged. Debbie Nuby has provided great competence in creating the graphics, tables, and manuscript, all of which have been essential to this study.
Dr. Thomas L. Spray (St. Louis, Mo.).
Could you elaborate about the measurements of important obstructive lesions that allowed you to classify the patients into the various classes of hypoplastic left heart syndrome. You noted, I think, that there were several patients who had Z-values of the subaortic diameter of more than six or seven and yet those patients were still classified as class I (no important obstruction).
How do you define obstruction? Is it only by echocardiographic evidence of a gradient, or is there no meaningful diagnosis of subaortic obstruction in these patients?
Dr. John W. Hammon, Jr. (Winston-Salem, N.C.).
In a patient who has IAA and subaortic stenosis as the only other important lesion other than VSD, can we essentially ignore the size of the subaortic area and do an appropriate operation, expecting that the subaortic stenosis will dilate or the patient will grow out of it? Is that a valid conclusion?
Dr. Michel N. Ilbawi (Oak Lawn, Ill.).
I find it very hard, Dr. Jonas, to understand why a simple myectomy or myotomy should adversely affect the outcome of these patients. It is a rather simple technique. I suspect that most of the patients who underwent this type of approach had a very significant subaortic stenosis, which was either not treated appropriately or was partially treated. Would you like to comment on that, please?
Dr. Jonas.
I think how one defines important obstruction really is the key issue here. We spent a lot of time grappling with exactly this issue. Unfortunately, the categorization into hypoplastic left heart class is to some extent subjective. Our hope was that something objective like the echocardiographically determined subaortic diameter would correlate with hypoplastic left heart class.
Not only could we not get a correlation between that dimension and hypoplastic left heart class, but we also could not get a correlation between measurements done by two independent expert observers as well as between those two observers and the dimension determined by the referral institution. Thus it will be important in the future to standardize echocardiographic technique so that we may be able to define in morphologic terms what important obstruction is.
In terms of ignoring the subaortic stenosis, that is essentially what it comes down to. All these patients do have a small subaortic region.
There is evidence that in some patients the subaortic dimension may enlarge after repair, after VSD closure. Perhaps there is a dynamic component so that when left ventricular pressure is greater than right ventricular pressure and a patch is anchored to the conal septum, the septum may partially move out of the way. Nevertheless, there is a biologic spectrum, and there are patients who have a 1 mm diameter subaortic region. At some point, therefore, you will have to depart from a standard repair.
The Damus-Kaye-Stansel approach does not appear to be the right thing to do at the severe end of the spectrum. Remember that this is not just a Norwood operation because it also involves reconstruction of the interrupted aortic arch. Our experience with the Norwood procedure for aortic atresia with IAA is that this has carried a 100% mortality.
Perhaps heart transplantation should be considered when the subaortic region is virtually occluded. However, for the vast majority of patients the subaortic stenosis can be ignored, although this analysis would suggest that the anastomotic area should be augmented with homograft or pericardium. I do not understand why that should help an upstream obstructive problem, but that is what this analysis implies.
I do not know why the patients having myotomy and myectomy patients did not do well in this analysis. It is not simply because these were higher risk patients because this came from a multivariable analysis that included morphologic and procedure-related variables.
Dr. Edward L. Bove (Ann Arbor, Mich.).
Did you say that, when you evaluated the Z-values for the subaortic area the vast majority had an absolute dimension of 4 mm or more? How did the Z-value correlate with the absolute dimension in millimeters?
Dr. Jonas.
No. The Z-value was less than -4 in almost all patients and in some patients was as small as -10. Although I do not have the corresponding absolute dimensions for the subaortic diameter, I can tell you that in the case of the left ventricleaorta junction diameter, a Z-value of -4 in the average neonate corresponds to a diameter of 5.9 mm, and a Z-value of -9 corresponds to 3.2 mm.
Dr. Bove.
I entirely agree with the attempt to put these data into a standardized measurement by using the Z-value. We reported to this Association last year a small series of seven patients who had this anatomy, all of whom had a subaortic myectomy at the time of primary repair but none of whom had an absolute dimension greater than 3.9 mm; most were between 2 and 3.5 mm. I am trying to determine if we are talking about the same type of patients.
Dr. Jonas.
The range of Z-values extended from -4 to about -10 corresponding to an absolute dimension as small as 3 to 3.5 mm.
Dr. Bove.
Do any of your data suggest that, although mortality was unaffected, the patients with the smaller Z-values had residual subaortic stenosis that was more important or needed earlier reintervention?
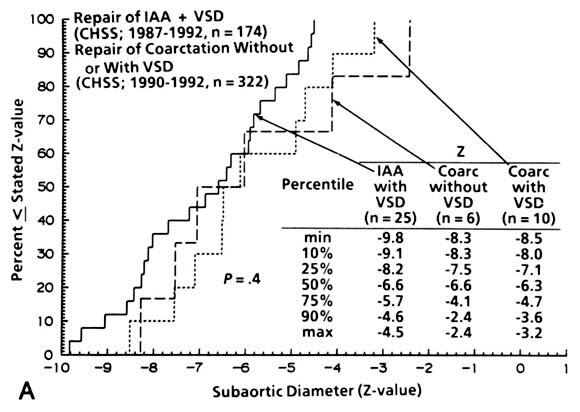
| Appendix Fig. 1. The Z-value for the institutionally measured diameters of (A) subaortic area (n = 31), (B) "anulus" (n = 112), and (C) ascending aorta (n = 127) in patients with IAA and VSD and coarctation with and without VSD. CHSS, Congenital Heart Surgeons Society. |
 |
Dr. Jonas.
I do not believe the data would support that conclusion, although intuitively that would seem to be likely.
Potential risk factors were entered simultaneously into multiple multivariable time-related analyses for the outcome events (1) death, (2) reintervention to the arch repair, and (3) reintervention against coexisting levels of obstruction in the left heartaorta complex. The hazard function regression model was used, 6 and an iteratively determined P value of less than 0.1 (because of the small size of the sample) was used as the criterion for including a variable in the final equation. Numerous transformations and interactions were explored as part of the analyses. The following variables were entered:
Demographic: Gender; birth weight; age; height; weight; body surface area at entry; age at repair
Prerepair treatment: Use of prostaglandin E1; intubation; catecholamines
Patient-specific cardiac variables: Type of IAA; (one versus more than one) of VSDs; size of VSD; concomitant obstructive lesions in the left heartaorta complex; anomalous origin of right subclavian artery; left superior vena cava; DiGeorge syndrome
Noncardiac congenital anomaly
Echocardiographic measurements (Z-value): Institutional measurement of diameter of subaortic region; left ventricularaortic junction (anulus); and ascending aorta
Procedural: Interval between entry and repair; technique of arch repair; one-stage initial repair; initial IAA repair and pulmonary trunk banding; initial IAA repair without closure of VSD or pulmonary trunk banding; concomitant ascending aorta/arch augmentation, subaortic myotomy/ myectomy, Damus-Kaye-Stansel anastomosis or other pulmonary trunkascending aorta anastomosis; use of systemicpulmonary artery shunt
Institutional and experience: Each institution; date of entry; date of initial repair; case sequence number (number of cases)
To maximize the knowledge obtained through the process of multivariable analysis, we grouped the variables and conducted the analyses sequentially. Into the first set of analyses, the patient-specific variables including demographic and morphologic ones and the dimensions were entered. Among the morphologic variables were any coexisting obstructions at the other individual levels in the left heartaorta complex. The second set of analyses examined institutional and experience variables, as well as patient-specific ones. As the institutions were entered into the analysis, the presence, coefficients, and P values of the previously retained patient-specific variables were allowed to change. The third set of analyses examined in the same manner the combination of patient-specific and procedural risk factors. A fourth set of analyses similarly examined patient-specific, procedural, and institutional and experience potential risk factors. These analyses allowed, among other things, inferences as to the role of suboptimal protocols on institutional performance.
The small numbers of patients and/or events in some institutions posed special problems in the multivariable analyses into which institutions were entered, which were handled as follows. After the multivariable equation with patient-specific risk factors only had been finalized, work was begun on an equation potentially containing institutions also as risk factors, using the Q-statistic. As is usually done, the Q-statistics were determined noniteratively and simultaneously as initial estimates of the strength (approximate coefficient) and believability (statistical significance) (P values) of each of the potential explanatory variables (such as institutions) as risk factors. When the approximate P value is large (>0.20) and the approximate coefficient small (<1), it is unlikely that the variable (such as an institution) adds anything more than could be expected from chance alone and it is rejected as a potential risk factor. A small number of institutions appeared to add information and were not rejected. Attempts were then made iteratively to determine exact coefficients and P values for these institutions (or other variables). In this study, one or two of the institutions were computationally intractable, because of only one or two or three patients or outcome events. Such institutions were not retained in the equation but were considered to be possible risk factors.

Copyright © 1994 by MosbyYear Book, Inc.