
 |
|
|
|
|
Jan M. Quaegebeur, MDa [MEDLINE LOOKUP]
Richard A. Jonas, MDb [MEDLINE LOOKUP]
Alan D. Weinberg, MSa(by invitation) [MEDLINE LOOKUP]
Eugene H. Blackstone, MDc [MEDLINE LOOKUP]
John W. Kirklin, MDc [MEDLINE LOOKUP]
the Congenital Heart Surgeons Society
New York, N.Y., Boston, Mass., and Birmingham, Ala.

Patients and Methods 
Results 
Discussion

Among 326 severely symptomatic neonates with coarctation with or without ventricular septal defect, four died before an initial procedure was performed. Among the 322 undergoing an initial procedure, survival for at least 24 months was 84%; the hazard function for death was lower initially but more prolonged in patients without than in those with ventricular septal defect. Important mitral valve anomalies coexisted in 5% of patients, left ventricular hypoplasia in 5% (more commonly in patients without ventricular septal defect), narrowing of the left ventricular outflow tract in 9% (more common in patients without ventricular septal defect), and narrowing of the proximal arch in 1%; one or more of these anomalies was present in most patients without ventricular septal defect who died. Five percent of the 322 patients had more than one of these coexisting anomalies, and 8% had just one. The most commonly used technique of repair of the coarctation was resection and end-to-end anastomosis, but no technique was a risk factor for death by multivariable analysis. Extension of the area of resection so that the end-to-end anastomosis was proximal to the left subclavian artery but distal to the left common carotid artery did not increase risk; extensions beyond this, and in the case of patch graft repair, extensions proximal to the left subclavian artery, did increase risk. Patch graft repair was associated with the highest prevalence (21%) of reintervention to the coarctation repair. Among patients with coexisting moderate-sized or large ventricular septal defects, repair of the coarctation, pulmonary trunk banding, and subsequent repair of the defect were associated with the highest 2-year survival, 97% in those with single ventricular septal defect. The risk-adjusted outcomes in two institutions were less good than in all others. (J THORAC CARDIOVASC SURG 1994;108:841-54)
Patients
This study addresses the 326 neonates with coarctation without (n = 171) or with (n = 155) VSD, who are among the 435 neonates with coarctation entering the 27 institutions between January 1, 1990, and January 1, 1992. The remainder (n = 109) were neonates in whom coarctation coexisted with other major congenital cardiac anomalies (Appendix Table I).
| Total deaths | |||||
| Coexisting cardiac anomalies | n | % of 432 | No. | % | 70% CL (%) |
| None | 171 | 40 | 23 | 13 | 11-17 |
| VSD (isolated) | 155 | 36 | 26 | 17 | 14-20 |
| Single ventricle* | 32 | 7 | 22 | 69 | 58-78 |
| TGA | 27 | 6 | 5 | 19 | 11-29 |
| AV canal! | 16 | 4 | 9 | 56 | 40-71 |
| DORV | 9 | 2 | 1 | 11 | 1-33 |
| Taussig-Bing heart | 12 | 3 | 6 | 50 | 32-68 |
| CCTGA | 6 | 1 | 2 | 33 | 12-62 |
| Truncus arteriosus | 1 | 0.2 | 1 | 100 | 15-100 |
| Anomalous origin of LCA from PT | 1 | 0.2 | 1 | 100 | 15-100 |
| TAPVC (with VSD) | 1 | 0.2 | 0 | 0 | 0-85 |
| PAPVC | 1 | 0.2 | 1 | 100 | 15-100 |
| Subtotal | 432 | 100 | 97 | 22 | 20-25 |
| Unknown | 3 | 0 | 0 | 0-47 | |
| Total | 435 | 100 | 97 | 22 | 20-25 |
AV, Atrioventricular; CCTGA, congenitallycorrected transposition of the great arteries; DORV,double-outlet right ventricle; LCA, left coronary artery;LV, left ventricle; PAPVC, partial anomalouspulmonary venous connection; PT, pulmonary trunk; RV, right ventricle; TAPVC, total anomalous pulmonary venousconnection; TGA, transposition of the great arteries; VS, ventricular septum; VSD, ventricular septal defect. |
|||||
*Univentricular atrioventricular connection (double-inlet left ventricle in 12; double-inlet right ventricle in one; mitral atresia in13; tricuspid atresia in five; common ventricle in one). |
|||||
Intact ventricular septum in three; VSD in 24. |
|||||
!Complete in 14; partial in two. |
|||||
Management
Most patients entered critically ill, and treatment was begun immediately with prostaglandin E1, intubation and ventilation, and catecholamines when indicated.2 Diagnosis was established quickly, usually by two-dimensional echocardiography but at times by cardiac catheterization and cineangiography. Management was a joint effort of the pediatric cardiology and pediatric cardiac surgery departments and involved other groups of physicians as well. Generally, a procedure was accomplished as soon as good hemodynamic and ventilatory states were achieved; in some patients, however, treatment could not achieve this, and a procedure was undertaken with the neonate in an unstable and unsatisfactory condition.
Follow-up
A cross-sectional follow-up of all patients in the study was undertaken annually between March 1 and May 1, and the last follow-up period from which data for this study were taken was in 1992. Eighty percent of the patients were followed by the Data and Analysis Center, and the remainder were followed by the treatment institution, at the institutions request.
The median follow-up time for survivors was 13.3 months, and the range was 3 days to 32.4 months; the mean follow-up time was 14.0 ± 7.50 months (standard deviation). Ninety percent of the patients were observed for 5.2 months or longer, 75% for 7.8 months or longer, 50% for 13.3 months or longer, 25% for 20.3 months or longer, and 10% for 24.3 months or longer. Nine patients have been untraceable since the time of hospital discharge.
Database
Copies of the relevant parts of the hospital records were provided to the Data and Analysis Center in the Division of Cardiothoracic Surgery at the University of Alabama at Birmingham. The patient-specific information was entered into a computer database and rechecked and upgraded on numerous occasions. Both noncomputerized and computerized files were treated in a private and confidential manner.
Analysis
A dataset for analysis was created from the computer database, using SAS System software (SAS Institute, Inc., Cary, N.C.) and an IBM RISC 6000 computer. Numerous explorations were made using Kaplan-Meier time-related nonparametric estimations,3 cumulative frequency distribution plots, and contingency tables. P values were estimated, and the method (model) is given wherever these are shown. Seventy percent confidence intervals were computed for most proportions, including time-related proportions, using these rather than 95% intervals to avoid overlooking possibly important differences in this rather small sample of 322 patients. Survival and hazard functions were also estimated parametrically, by means of the hazard function regression model.4
Numerous multivariable analyses of time-related determined outcomes (hazard function regression domain), many more than are presented in this publication, were performed with a P value of less than 0.1 used as the criterion for retaining a variable in the final equation, with the same reasoning as for 70% confidence intervals. However, the P values for most retained variables were considerably smaller than this. Potential risk factors (Appendix Table 2) were entered sequentially in groups (groups of patient-specific, procedural, and institutional variables).
| Demographic: Gender; birth weight; age, height, weight, body surface area at entry; age at repair |
| Prerepair treatment: Use of prostaglandin E1; intubation; catecholamines |
| Cardiac variables: Patency of ductus arteriosus; amomalous origin of LSA; multiplicity of VSD if present; size of VSD (0, small, medium, large); coexisting obstructive lesions in the left heart-aorta complex (mitral valve anomalies, left ventricular hypoplasia, left ventricular outflow narrowing [subaortic region, left ventricular-aortic junction ("annulus"), aortic valve], ascending aorta [including sinuses of Valsalva and sinotubular junction], and proximal aortic arch [between IA and LCC]) |
| Noncardiac congenital anomaly |
| Echocardiographic measurements (transformed to Z-value): Diameters of subaortic channel, left ventricular-aortic junction ("annulus"), and ascending aorta |
| Procedural: Interval between entry and procedure; type of initial procedure; technique or coarctation repair; proximal extent of patch graft or end-to-end anastomosis |
| Institutional: Each instution; date of entry; date of initial repair; case sequence number (number of cases) |
IA, Innominate artery;LCC, left common carotid artery;LSA, left subclavian artery;VSD, ventricular septal defect. |
Note: The distal aortic arch (between the LCC and the attachment of the ductus arteriosus to the thoracic aorta) was not entered as this is considered part of the coarctation (or interrupted arch). |
 2) value. In the other method, the time-related freedom from the event was determined by the Kaplan-Meier method. This was compared with the percent freedom from the event obtained by averaging the individual time-related freedoms of each patient; the comparison was considered satisfactory when the 70% confidence limits of the estimates were overlapping.
2) value. In the other method, the time-related freedom from the event was determined by the Kaplan-Meier method. This was compared with the percent freedom from the event obtained by averaging the individual time-related freedoms of each patient; the comparison was considered satisfactory when the 70% confidence limits of the estimates were overlapping.
Definitions
The presence, size (small, moderate-sized, or large, aggregate size in the case of multiple VSDs unless stated otherwise), multiplicity, and location of VSDs was judged from echocardiographic, angiographic, and operative reports. Coexisting lesions in the left heartaorta complex (listed in Appendix Table 2) were subjectively graded as to the severity of their obstructiveness from 0 to 5 (5 being the most severe) on the basis of these documents and any available echocardiographic measurements; grade 2 or more was considered to represent a functionally important degree of obstruction. This method was used because of the paucity of echocardiographic measurements made by the institutions and because of the inappropriateness of inferences about obstruction based on gradients across these areas in neonates with patency of the ductus arteriosus and/or a VSD.
Classic coarctation repair is defined in this study as one (end-to-end anastomosis, subclavian flap, or patch graft) that does not extend proximal to the left subclavian artery. Even though hypoplasia of that portion of the distal arch between the left common carotid artery and the left subclavian artery is not considered a coexisting obstructive anomaly because of clear evidence of its reversibility,6 the repair was considered to augment the diameter of the arch when it extended proximal to the left subclavian artery.
Hospital deaths are included, as well as deaths at any time in the follow-up period. Time zero was usually the time of initial repair. The 30-day mortality is evident in all the time-related depictions.
Non-risk-adjusted prevalences and percents of death (or survival) and other events are those that were obtained by simply counting and dividing, or by actuarial (Kaplan-Meier) analysis. Risk-adjusted prevalences and percents are those in which the values for all variables except the one(s) under consideration are kept constant; that is, the effect of the variable(s) under consideration is isolated from the effect of any other variables and thus the risk associated with it per se is more clearly evident. Risk-adjusted prevalences are valuable in drawing inferences, but there is a degree of uncertainty (quantified by confidence intervals and P values) in their values.
Prevalences
The median birth weight at entry was 2.97 kg (90% between 1.61 and 4.04 kg). The median age at entry was 6 days (90% were between 0 and 23 days old). The median interval between entry and the first procedure was 3 days (90% were between 0 and 17 days).
Obstruction was present at levels in the left heartaorta complex other than at the coarctation in 13% of the patients ( Table I).
| Total deaths | |||||
| Coexisting obstructive lesions* | n | % of 322 | No. | % | 70% CL (%) |
| None | |||||
| Coarctation only, without or with VSD | 280 | 87 | 22 | 8 | 6-10 |
| One additional left heart-aorta anomaly | 25 | 7.8 | 10 | 40 | 29-52 |
| MV anomaly | 7 | 4 | 57 | 32-80 | |
| LVOTO | 15 | 5 | 33 | 19-50 | |
| Hypoplasia of aortic arch (between IA and LCC artery) | 3 | 1 | 33 | 4-76 | |
| Two or more additional left heartaorta anomalies | 17 | 5.3 | 15 | 88 | 74-96 |
| Total | 322 | 100% | 47 | 15 | 13-47 |
P( 2) 2) |
<0.0001 | ||||
CL, Confidence limits; IA, innominate artery; LCC, left common carotid artery; LVOTO, left ventricular outflow tract obstruction; MV, mitral valve; VSD, ventricular septal defect. |
|||||
*In toto, mitral valve anomaly was present in 17 (5%) patients (14 of whom died); left ventricular hypoplasia in 15 (5%) (13 of whom died); LVOTO, either subvalvular in eight (2%) (seven of whom died), or valvular in 20 (6%) (nine of whom died); and ascending aorta/archhypoplasia proximal to the left common carotid artery in four (1%) (one of whom died). Hypoplasia of the left ventricular-aortic junction ("anulus") is assumed to coexist when subvalvular narrowing is present in this condition, but is not separately categorized. |
|||||
Among the 17 patients, in toto, with mitral valve anomaly, in eight the mitral valve had a single (n = 6), or essentially single (n = 2), papillary muscle (parachute mitral valve); in two the mitral valve had short abnormal chordae resulting in a narrow orifice; in one the mitral valve had short abnormal chordae resulting in an incompetent valve (a supraannular ring coexisted but did not produce stenosis); in one it had thickened stenotic leaflets; and in five it was hypoplastic and stenotic related to the small diameter of the anulus. (Not included in this tabulation are 13 patients whose mitral valve had a single papillary muscle but functioned normally.) |
|||||
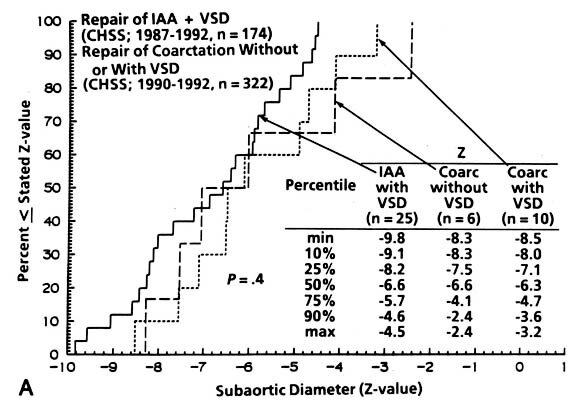
| Fig. 1. The Z-value for the institutionally measured diameters of (A) subaortic area (n = 31), (B) "anulus" (n = 127), and (C) ascending aorta (n = 55) in neonates with coarctation with and without VSD and in patients with interrupted aortic arch and VSD. IAA, Interrupted aortic arch; LV, left ventricle; VSD, ventricular septal defect; CHSS, Congenital Heart Surgeons Society. (Reproduced from Jonas et al.7) |
 |
 |
 |
No VSD was present in 169 (52%) of the 322 patients. The VSD was small in 52 patients, moderate-sized in 45, and large in 52; 25 of the 97 moderate-sized or large VSDs were multiple ( Table II).
| Total deaths | |||||
| Type of VSD | n | No | % | 70% CL (%) | |
| No VSD | 169 | 22 | 13 | 10-16 | |
| Small | 52 | 6 | 12 | 7-18 | |
| Single | 40 | 5 | 12 | 7-20 | |
| Multiple | 12 | 1 | 8 | 1-26 | |
| Moderate-sized | 45 | 7 | 16 | 10-23 | |
| Single | 30 | 4 | 13 | 7-23 |  |
| Multiple | 15 | 3 | 20 | 9-36 | |
| Large | 52 | 10 | 19 | 13-27 | |
| Single | 42 | 6 | 14 | 9-22 |  |
| Multiple | 10 | 4 | 40 | 22-61 | |
| VSD size unknown | 4 | 2 | |||
P( 2) 2) |
0.6 | ||||
| Total | 322 | 47 | 15 | 13-17 | |
Survival
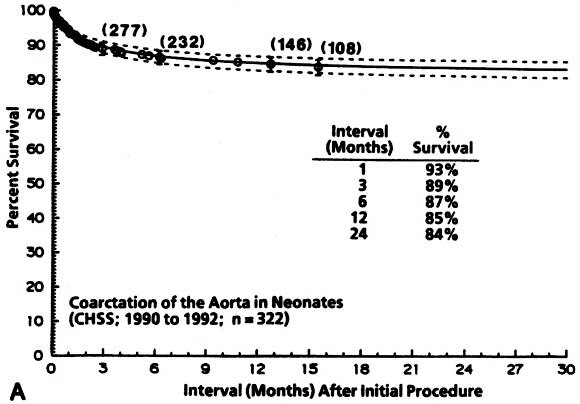
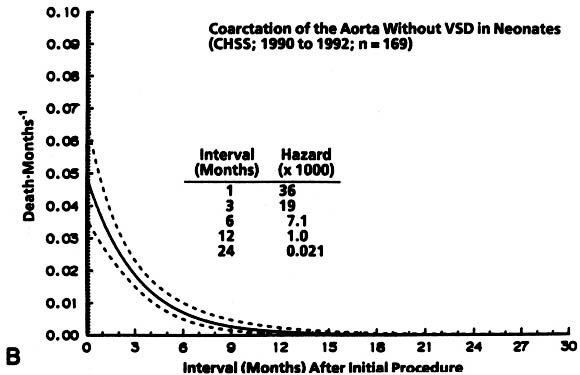
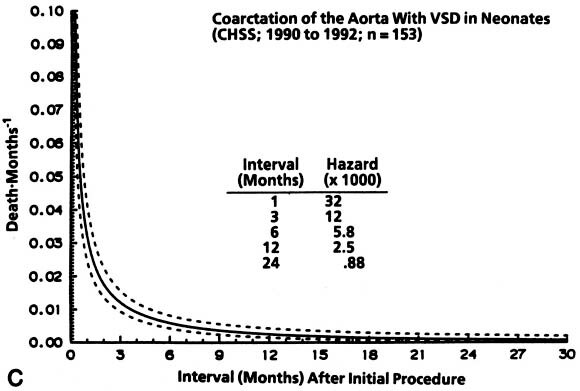
Non-risk-adjusted survival for at least 1 month among the 322 patients was 93%, and it was 84% for 24 months. The hazard function for death for coarctation without VSD was lower initially and more prolonged than that for coarctation with VSD ( Fig. 2).
| Fig. 2. Survival and hazard function for death after the initial intervention (at time zero) in neonates with coarctation with and without VSD (n = 322). A, Survival. Each circle represents an actual death, positioned at the time of death along the horizontal axis and actuarially (Kaplan-Meier) along the vertical axis. The vertical bars depict the 70% confidence limits (±1 standard error) of the Kaplan-Meier actuarial estimate. The numbers indicate the number of patients remaining at risk at the time of the estimate. The solid line is the parametric estimate of survival, and the dashed lines enclose the 70% confidence intervals. (The similarity of the two is a validation of the hazard function method.) B, Hazard function for death in patients without VSD. C, Hazard function for death among patients with VSD. CHSS, Congenital Heart Surgeons Society. |
 |
 |
 |
A single moderate-sized or large VSD slightly, but not believably, decreased the non-risk-adjusted survival after repair (see Table II,
Appendix Fig. 1) and believably decreased the risk-adjusted survival (
Table III).
| Single hazard phase | ||
| Incremental risk factors for death (patient-specific and procedural variables) | Coefficient ± SD | P value |
| Demographic | ||
| Age (days)* at repair and multiplicity of VSDs (interaction term) | 0.33 ± 0.071 | <0.0001 |
| Morphologic | ||
| Single, moderate-sized or large VSD | 1.16 ± 0.47 | 0.01 |
| Size of VSDs** and multiplicity of VSDs (interaction term) | 3.9 ± 1.48 | 0.009 |
| Multiplicity of VSDs | 6.6 ± 1.57 | <0.0001 |
| MV anomaly (without or with other anomalies of the left heart-aorta complex) | 3.7 ± 0.43 | <0.0001 |
| Subaortic narrowing and no mitral valve anomaly | 3.8 ± 0.58 | <0.0001 |
| Aortic valve stenosis, isolated | 1.94 ± 0.67 | 0.004 |
| Aortic valve stenosis and LV hypoplasia (interaction term) | 3.2 ± 0.79 | <0.0001 |
| Severe noncardiac anomalies | 1.17 ± 0.54 | 0.03 |
| Procedural ! | ||
| Extension of end-to-end repair proximal to LCC artery, and VSD (interaction term) | 1.45 ± 0.56 | 0.009 |
| Extension of patch graft proximal to LSA, and moderate-sized or large VSD (interaction term) | 1.87 ± 0.47 | <0.0001 |
| Repair of coarctation with PT band and presence of VSD (interaction term) | 1.55 ± 0.67 | 0.02 |
Intercept = 3.125;  = 6.954; = 6.954;  = 0; m = 1.511; log likelihood = 155.4603. LCC, Left common carotid; LSA, left subclavian artery; LV, left ventricular; MV, mitral valve; PT, pulmonary trunk;VSD,ventricular septal defect. Note: (1) Retained left heart-aorta complex variables in this and all other equations aregrade 2 or greater in severity. (2) All variables, in this and other depictions of multivariable equations, are dichotomous (yes/no) except*indicates continuous variables and **indicates ordinal variables. (3)The negative coefficient for "size and multiplicity of VSDs" is related to the inverse transformation used; that for "repair of coarctation with PT band and presence of VSD" is related to this variable being associated with a lower risk. = 0; m = 1.511; log likelihood = 155.4603. LCC, Left common carotid; LSA, left subclavian artery; LV, left ventricular; MV, mitral valve; PT, pulmonary trunk;VSD,ventricular septal defect. Note: (1) Retained left heart-aorta complex variables in this and all other equations aregrade 2 or greater in severity. (2) All variables, in this and other depictions of multivariable equations, are dichotomous (yes/no) except*indicates continuous variables and **indicates ordinal variables. (3)The negative coefficient for "size and multiplicity of VSDs" is related to the inverse transformation used; that for "repair of coarctation with PT band and presence of VSD" is related to this variable being associated with a lower risk. |
||
The technique of the repair of the coarctation was not found to be abelievable risk factor. |
||
!"Balloon dilation of coarctation" was suggested by Q-statistics to be a believable risk factor, but was computationally intractable to iterative analysis in this and subsequent analyses. It is possibly a risk factor for death. |
||
The surgical technique for repair of the coarctation did not affect the non-risk-adjusted ( Table IV) nor the risk-adjusted (see
Table III) survival so long as the repair was not extended proximal to the left subclavian artery.
| Death | Reinterventions | |||||||
| Technique of coarctation repair | n | % of 319 | No. | % | 70% CL (%) | No. | % | 70% CL (%) |
| End-to-end anastomosis | 139 | 44 | 20 | 14 | 11-18 | 6 | 4 | 3-7 |
| Distal to or at LSA ("classic") | 27 | 8 | 3 | 11 | 5-21 | 1 | 4 | 0.5-12 |
| Proximal to LSA distal to LCC | 92 | 29 | 12 | 13 | 9-18 | 5 | 5 | 3-9 |
| Proximal to LCC distal to IA | 8 | 2 | 3 | 38 | 17-62 | 0 | 0 | 0-21 |
| Proximal to IA | 3 | 1 | 2 | 67 | 24-96 | 0 | 0 | 0-47 |
| Unknown | 9 | 3 | 0 | 0 | 0-19 | 0 | 0 | 0-19 |
| Subclavian flap ("classic") | 112 | 35 | 9 | 8 | 5-12 | 6 | 5 | 3-9 |
| Patch graft | 38 | 12 | 11 | 29 | 21-38 | 8 | 21 | 14-30 |
| Extending to LSA (classic) | 15 | 5 | 2 | 13 | 4-29 | 4 | 27 | 14-43 |
| Extending to LCC | 7 | 2 | 2 | 29 | 10-55 | 2 | 29 | 10-55 |
| Extending to IA | 1 | .3 | 0 | 0 | 0-85 | 0 | 0 | 0-85 |
| Extending to ascending aorta | 12 | 4 | 6 | 50 | 32-68 | 2 | 17 | 6-35 |
| Extension unknown | 3 | 1 | 1 | 33 | 4-76 | 0 | 0 | 0-47 |
| Left common carotid flap | 1 | 0.3 | 1 | 100 | 15-100 | 0 | 0-85 | |
| End-to-end plus subclavian flap ("classic") | 15 | 5 | 1 | 7 | 1-21 | 2 | 13 | 4-29 |
| End-to-end plus patch graft ("classic") | 2 | 0.6 | 0 | 0 | 0-61 | 0 | 0 | 0-61 |
| End-to-end plus reverse subclavian flap | 5 | 2 | 1 | 20 | 3-53 | 0 | 0 | 0-32 |
| End-to-end plus reverse subclavian flap plus patch graft | 1 | 0.3 | 0 | 0 | 0-85 | 0 | 0 | 0-85 |
| Reverse subclavian flap plus patch graft | 2 | 0.6 | 0 | 0 | 0-61 | 0 | 0 | 0-61 |
| Subclavian flap ("free")plus LCC flap | 1 | 0.3 | 0 | 0 | 0-85 | 0 | 0 | 0-85 |
| Percutaneous balloon dilation of coarctation | 3 | 1 | 2 | 67 | 24-96 | 2 | 67 | 24-96 |
| Subtotal | 319 | 100 | 45 | 14 | 12-16 | 24 | 8 | 6-9 |
| Repair of coarctation by unknown method | 3 | 2 | 0 | |||||
| Total | 322 | 100 | 47 | 15 | 13-17 | 24 | 7 | 6-9 |
CL, Confidence limits; IA, interrupted arch; LCC, left common carotid artery; LSA, left subclavian artery. |
||||||||
Note: Four additional patients had reintervention to the coarctation repair at the time of repair of a VSD, but the restenosis was only mild. |
||||||||
When patch grafting was used as the technique of repair and was similarly extended proximally (that is, only as far up as the level of the left common carotid artery), the non-risk-adjusted survival was not believably decreased compared with that of patch graft repair limited to the upper descending thoracic aorta (29%; 70% C1* 10% to 55% versus 13%; 70% CL 4%-29%, P(Fisher) = 0.4, see Table IV); the risk-adjusted survival was decreased by this extension of the patch graft technique (see
Table III,
Fig. 4). No matter what the technique, extensions proximal to the left common carotid artery were associated with decreased survival. Only eight patients (one death) had a reverse subclavian flap used as part of the initial repair of coarctation, so generalizations about survival with this technique are not possible.
Repair of the coarctation alone was performed for all patients without VSD and for most (49 of 52) with small VSD. Actuarial, non-risk-adjusted survival in this group was 87% at 15 months and beyond, and most of the deaths after hospital dismissal were associated with coexisting obstruction at other levels. Risk-adjusted survival for the subset of this group that was without coexisting obstructive anomalies of the left heartaorta complex was 95% at 24 months (see Fig. 3).